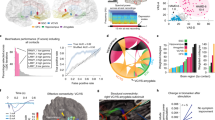
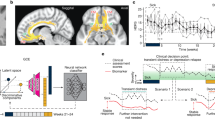
Abstract
Deep brain stimulation is a promising treatment for severe depression, but lack of efficacy in randomized trials raises questions regarding anatomical targeting. We implanted multi-site intracranial electrodes in a severely depressed patient and systematically assessed the acute response to focal electrical neuromodulation. We found an elaborate repertoire of distinctive emotional responses that were rapid in onset, reproducible, and context and state dependent. Results provide proof of concept for personalized, circuit-specific medicine in psychiatry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The source data that support the findings in this report are available in the report itself, in the Supplementary Information and in our publicly available code. Source data are provided with this paper.
Code availability
The code and data used to produce the figures in this paper are available at https://github.com/ScangosLab/PR01.
Change history
08 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41591-022-01950-9
References
Disease, G. B. D., Injury, I. & Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858 (2018).
Howland, R. H. Sequenced treatment alternatives to relieve depression (STAR*D). Part 2: study outcomes. J. Psychosoc. Nurs. Ment. Health Serv. 46, 21–24 (2008).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4, 839–849 (2017).
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78, 240–248 (2015).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 73, 456–464 (2016).
Scangos, K. W. & Ross, D. A. What we’ve got here is failure to communicate: improving interventional psychiatry with closed-loop stimulation. Biol. Psychiatry 84, e55–e57 (2018).
Mayberg, H. S., Riva-Posse, P. & Crowell, A. L. Deep brain stimulation for depression: keeping an eye on a moving target. JAMA Psychiatry 73, 439–440 (2016).
Malone, D. A. Jr. et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 65, 267–275 (2009).
Fraguas, D. et al. Mental disorders of known aetiology and precision medicine in psychiatry: a promising but neglected alliance. Psychol. Med. 47, 193–197 (2017).
Riva-Posse, P. et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 (2018).
Choi, K. S., Riva-Posse, P., Gross, R. E. & Mayberg, H. S. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72, 1252–1260 (2015).
Tharin, S. & Golby, A. Functional brain mapping and its applications to neurosurgery. Neurosurgery 60, 185–201 (2007).
Bishop, M. P., Elder, S. T. & Heath, R. G. Intracranial self-stimulation in man. Science 140, 394–396 (1963).
Rao, V. R. et al. Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr. Biol. 28, 3893–3902 e3894 (2018).
Kirkby, L. A. et al. An amygdala–hippocampus subnetwork that encodes variation in human mood. Cell 175, 1688–1700 (2018).
Scangos, K. W. et al. Pilot study of an intracranial electroencephalography biomarker of depressive symptoms in epilepsy. J. Neuropsychiatry Clin. Neurosci. 32, 185–190 (2020).
Timmerby, N., Andersen, J. H., Sondergaard, S., Ostergaard, S. D. & Bech, P. A systematic review of the clinimetric properties of the 6-item version of the Hamilton Depression Rating Scale (HAM-D6). Psychother. Psychosom. 86, 141–149 (2017).
Inman, C. S. et al. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia 145, 106722 (2018).
Machado, A. et al. Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clin. Neurophysiol. 120, 1941–1948 (2009).
Riva-Posse, P. et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 76, 963–969 (2014).
Campbell, M. C. et al. Mood response to deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 24, 28–36 (2012).
Salloum, N. C. et al. Efficacy of intravenous ketamine treatment in anxious versus nonanxious unipolar treatment-resistant depression. Depress. Anxiety 36, 235–243 (2019).
Mori, S. & van Zijl, P. C. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 15, 468–480 (2002).
Feffer, K. et al. 1Hz rTMS of the right orbitofrontal cortex for major depression: safety, tolerability and clinical outcomes. Eur. Neuropsychopharmacol. 28, 109–117 (2018).
Acknowledgements
This work was supported by National Institutes of Health award K23NS110962 (to K.W.S.), a NARSAD Young Investigator grant from the Brain and Behavioral Research Foundation (to K.W.S.) and the Ray and Dagmar Dolby Family Fund through the Department of Psychiatry at the University of California, San Francisco (to K.W.S., A.D.K., E.F.C. and L.P.S.). E.F.C. was supported by the National Institutes of Health (U01NS098971, R01MH114860, R01MH111444, R01DC015504, R01DC01237, UH3 NS109556, UH3NS115631 and R01 NS105675), the New York Stem Cell Foundation, the Howard Hughes Medical Institute, the McKnight Foundation, the Shurl and Kay Curci Foundation and the William K. Bowes Foundation. A.D.K. acknowledges support from the National Institutes of Health (P01AG019724, R01 HL142051-01, R01AG059794, R01DK117953, UH3 NS109556-01 and R01AG060477-01A1), PCORI, Janssen, Jazz, Axsome (AXS-05-301) and Reveal Biosensors. L.P.S. receives support from the National Institutes of Health (U24 DA041123).
Author information
Authors and Affiliations
Contributions
K.W.S., A.D.K. and E.F.C. initiated this work and supervised the study. K.W.S. drafted the manuscript, and G.S.M., K.W.S. and L.P.S. collected and analyzed the data. A.D.K. and E.G.C. finalized the manuscript. All authors approved this work and take responsibility for its integrity.
Corresponding author
Ethics declarations
Competing interests
A.D.K. consults for Eisai, Evecxia, Ferring, Galderma, Harmony Biosciences, Idorsia, Jazz, Janssen, Merck, Neurocrine, Pernix, Sage, Takeda, Big Health, Millenium Pharmaceuticals, Otsuka Pharmaceuticals, and Neurawell. All other authors declare no competing interests.
Additional information
Peer review information Jerome Staal and Kate Gao were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scangos, K.W., Makhoul, G.S., Sugrue, L.P. et al. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med 27, 229–231 (2021). https://doi.org/10.1038/s41591-020-01175-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-01175-8